Pharma & Medical Devices
ERP Solutions for Life Sciences & Medical Device Manufacturing
Life sciences and medical device manufacturers are dedicated to innovation, product quality and patient safety, but a tightening regulatory environment, increasing cost pressures and supply chain complexities are causing new challenges. Emerging global markets, quality initiatives, and increasing mergers and acquisitions activity add to the complexity of life sciences or medical device manufacturing and distribution. ERP solutions from QAD deliver the flexibility that manufacturers in the life sciences and medical device industries require
450+
Life Sciences Manufacturing Sites Live on QAD
100
Countries with QAD Life Sciences Customers
$968 Billion
Annual Revenue Generated by QAD Life Sciences Customers

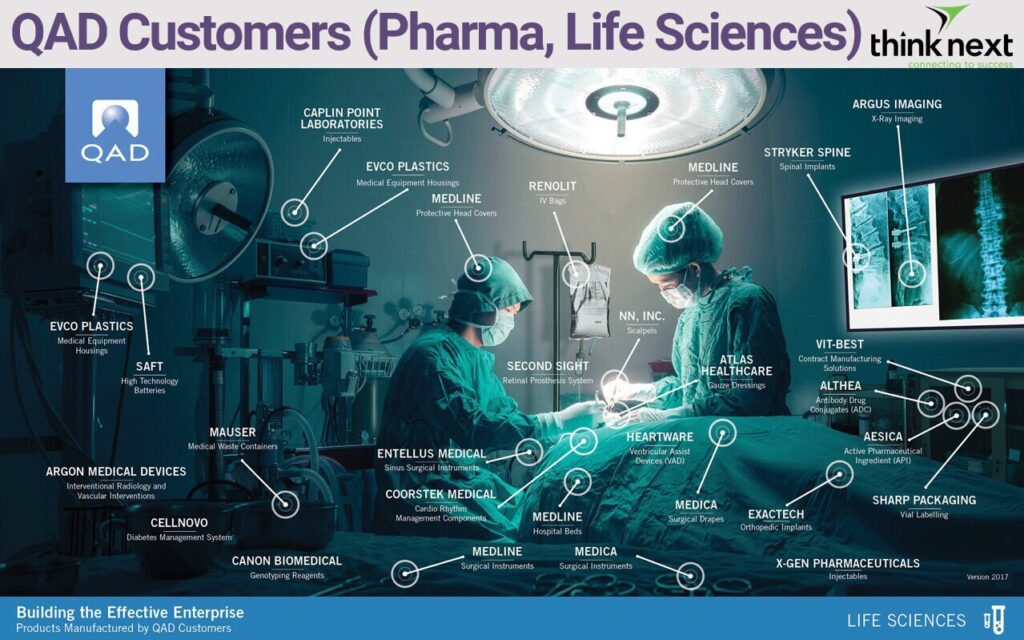
QAD has been a trusted solution partner to medical device manufacturers for decades:
- QAD Adaptive ERP is designed using industry best practices and provides a comprehensive yet flexible solution for global manufacturers
- Supply chain solutions offer end-to-end supply chain visibility with a shared view across manufacturing, including suppliers, customers, outsource partners and other trading partners
- Composable manufacturing solutions deliver vertically integrated quality, connected worker enablement, asset management and operational collaboration with planning
- Demand and supply chain planning drives inventory optimization and maximize production capacity
- QAD extensions of business systems deliver increased quality and productivity through connected workers and equipment to the shop floor
The Life Sciences Adaptive Enterprise
Business planning, operation and supply chain processes must be supported by composable and adaptable processes based on collective intelligence and responsiveness.
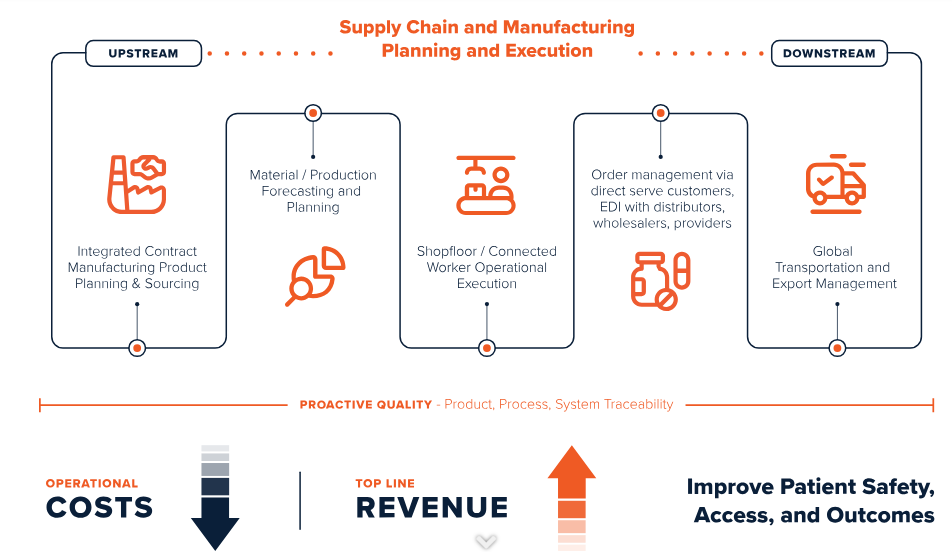
Smart Factory
Driving Business Impact in the Medical Devices and Pharma Industry
- Patient Focused – Manufacturers deliver safe and effective devices, diagnostics, and therapies that improve patient lives and experiences
- Quality First – Quality first initiatives enable compliance with applicable global regulations and requirements
- Innovative – Innovates to improve all aspects of the operation, supply chain, and product performance with a key focus on quality and traceability




